
最近,中国科学院过程工程研究所在燃料乙醇脱水新工艺的研究开发中获得新进展,主要研究工作A new process for fuel ethanol dehydration based on modeling the phase equilibria of the anhydrous MgCl2+ ethanol + water system 发表在化工国际期刊AIChE Journal (2015, 61, 664-676)上。
当今现代社会的发展过度依赖石油、煤炭和天然气等化石能源。这些化石能源是不可再生的,它们在使用过程中也产生了严重的环境问题,如二氧化碳和二氧化硫的排放等,因此迫切需要寻找新的替代资源。从玉米淀粉和糖类等生物物质通过生物技术制备的乙醇,是富有希望的化石能源替代品,由于燃料乙醇可以适用现有燃油发动机,从而可以方便地整合到燃油供应体系之中,现已得到大规模应用。目前,在美国汽油中掺有10%的燃料乙醇(E10),并计划将含量提高到15%。近日据路透社报导,巴西政府规定要将目前汽油中燃料乙醇的加入量从25%提高到27.5%。
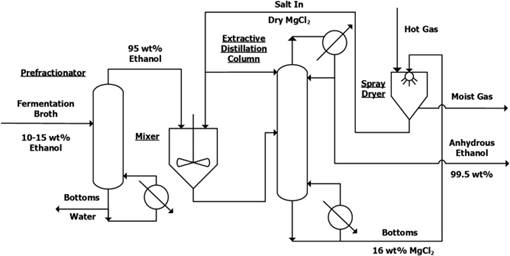
燃料乙醇的生产需要将生物发酵后得到的低浓度(<15%)乙醇进一步浓缩脱水为无水乙醇。由于乙醇—水体系存在共沸点且浓度超过共沸点后相对挥发度基本等于1,导致乙醇脱水80%的能量消耗,这是影响燃料乙醇大规模推广应用的主要瓶颈。绿色过程与工程实验室研究员李志宝和博士研究生(现已毕业)曾澜沐经过多年的实验和理论研究,提出了以氯化镁加盐精馏技术为基础的乙醇脱水新工艺(见下图)。实验发现氯化镁的加入可以显著提高乙醇—水体系的相对挥发度,并方便在工业上循环使用;通过引入Mg2+离子的水化反应,成功建立了该复杂体系的汽液平衡热力学模型。通过对新工艺的模拟计算,相比美国目前使用的分子筛法预计可降低约30%能量消耗。
以上研究工作受国家自然科学基金(21476235和21206165)、“973”计划(2013CB632605)以及青海科技厅项目(2012-G-213A)等的资助。(来源:中国科学院过程工程研究所)
A new process for fuel ethanol dehydration based on modeling the phase equilibria of the anhydrous MgCl2 + ethanol + water system
Abstract The use of ethanol as a fuel for motor engines has attracted significant attention because of its possible environmental and economic advantages over fossil fuel. However, the energy demand for the ethanol dehydration process significantly impacts its production cost. A new and energy efficient process is developed on the basis of salt extractive distillation, which uses recycled MgCl2 granules as a separating agent. Vapor-liquor-equilibria (VLE) data for the ternary MgCl2 + ethanol + water system, and the three constituent binary systems were measured at 30, 60, 90, and 101.3 kPa. A large enhancement of relative volatility of the ethanol + water system in the presence of MgCl2 is observed throughout the entire ethanol concentration range, which completely broke the azeotrope. The salt effect of MgCl2 is thought to be the result of energetic interactions and the hydration equilibrium reaction of the Mg2+ ion with water molecules. The calculation results by the mixed-solvent electrolyte model embedded in the OLI platform equipped with new model interaction parameters and equilibrium constant (obtained via the regression of experimental VLE data), provided for a satisfactory means of simulating the MgCl2 salt extractive distillation process. Finally, the process was proven feasible at the laboratory-scale resulting in large granules of recovered MgCl2 and a product of 99.5 wt % ethanol.
原文链接:http://onlinelibrary.wiley.com/doi/10.1002/aic.14685/pdf



